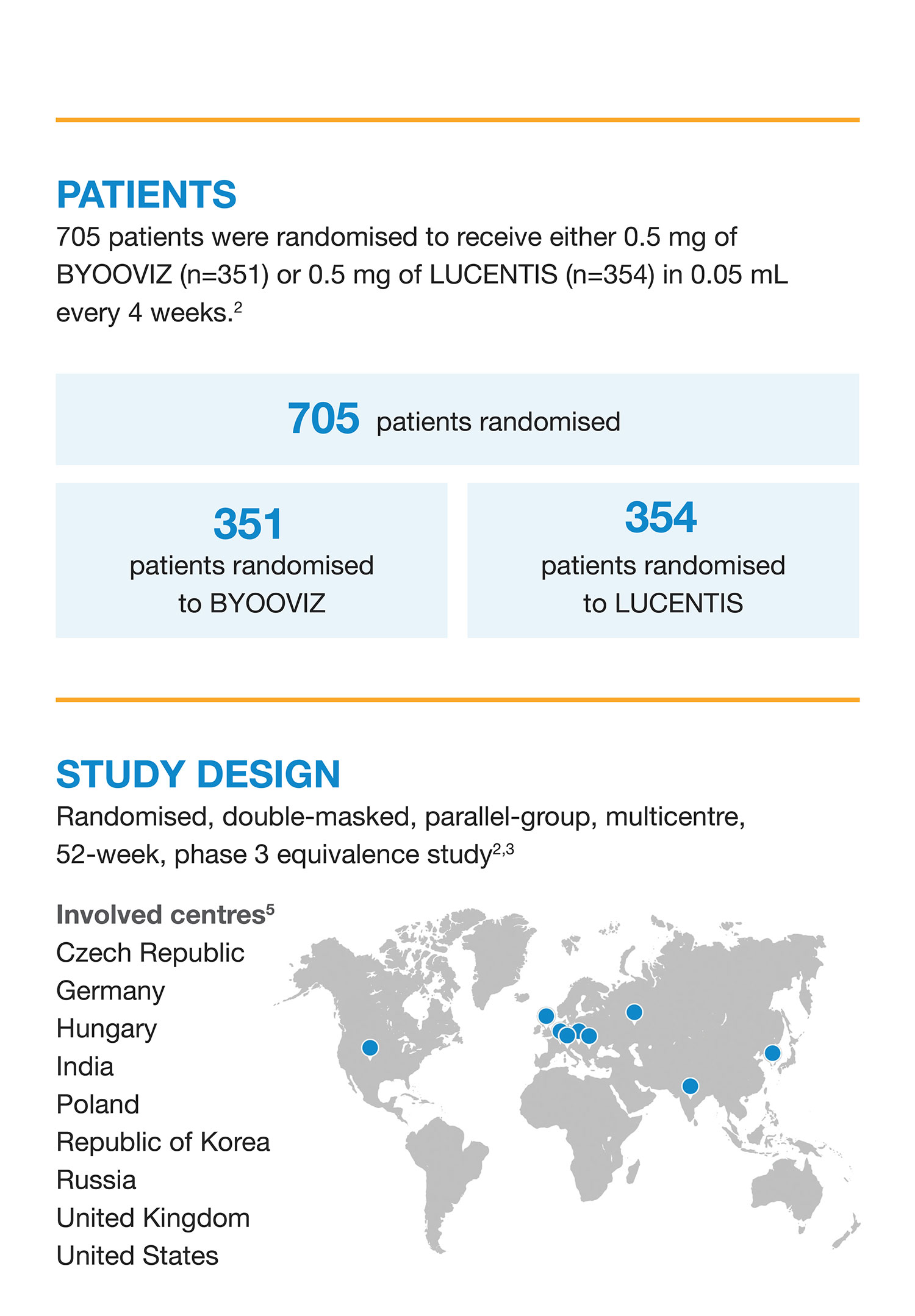
Is It Possible to Give More Patients With Retinal Vascular Disorders a Chance to See Their World?

A biosimilar* referencing PrLUCENTIS (ranibizumab injection) 1
Approved for the treatment of 1 :
nAMD DME CNV
macular edema
secondary to RVO
*A biosimilar biologic drug, or biosimilar, is a biologic drug that is highly similar to a biologic drug that was already authorised for sale. There are no expected clinically meaningful differences in efcacy and safety between a biosimilar and the biologic drug that was already authorised for sale.2 LUCENTIS is a trademark of Genentech, Inc. Indication and clinical use: BYOOVIZ (ranibizumab) is indicated for the treatment of: - neovascular (wet) age-related macular degeneration (AMD). - visual impairment due to: CNV=choroidal neovascularisation; DME=diabetic macular oedema; nAMD=neovascular age-related macular degeneration; RVO=retinal vein occlusion.
There’s So Much
More to See
References

Clinical Trial Results:
Similar Efficacy and
Comparable Safety Profile
to LUCENTIS1-4
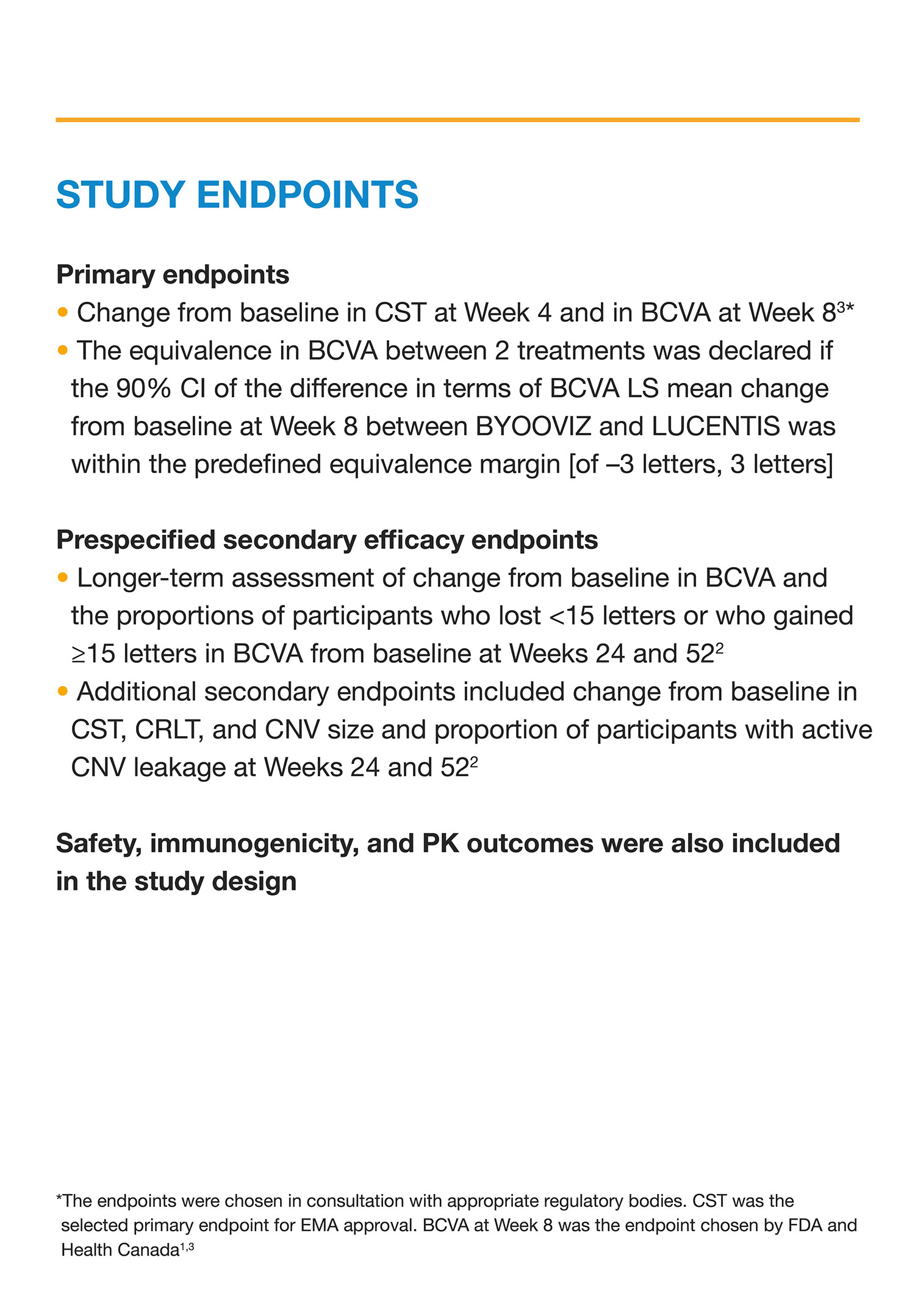
PRIMARY ENDPOINTS MET1,2
(Weeks 4 and 8)
PRIMARY ENDPOINTS MET1,2
(Weeks 4 and 8)
Change from baseline in:
- CST at Week 4
- BCVA at Week 8
BYOOVIZ met the primary endpoint in a phase 3 equivalence study1
- The treatment difference in BCVA of the change from baseline between BYOOVIZ and at Week 8 was letters and the 95% Cl was [-2.023, 0.415], which was contained within the pre-defined similarity margin of letters, [-3 letters, 3 letters]1
SECONDARY ENDPOINTS MET2,3
(Weeks 24 and 52)
SECONDARY ENDPOINTS MET1,2
(Weeks 24 and 52)
Change from baseline in:
- CST
- BCVA
- CRLT
- CNV size
- Active CNV leakage (proportion of patients)
SAFETY PROFILE COMPARABLE TO LUCENTIS1-4
(Most commonly reported adverse events)
SAFETY PROFILE COMPARABLE TO LUCENTIS1-4
The types, frequency, and severity of adverse events were comparable between BYOOVIZ and the reference product LUCENTIS1
- No definitive differences in the overall
incidence of AESIs between treatment groups
were identified2
- Increased intraocular pressure (BYOOVIZ,
3 [0.9%]; LUCENTIS,
6 [1.7%])2
- Increased intraocular pressure (BYOOVIZ,
3 [0.9%]; LUCENTIS,
Please refer to the BYOOVIZ Product Monograph for complete safety information.
AESI=adverse events of special interest. Where do you see
BYOOVIZ fitting into
your practice?
NEED MORE INFORMATION ABOUT:

There's So Much More to See

There's So Much More to See

There's So Much More to See

There's So Much More to See